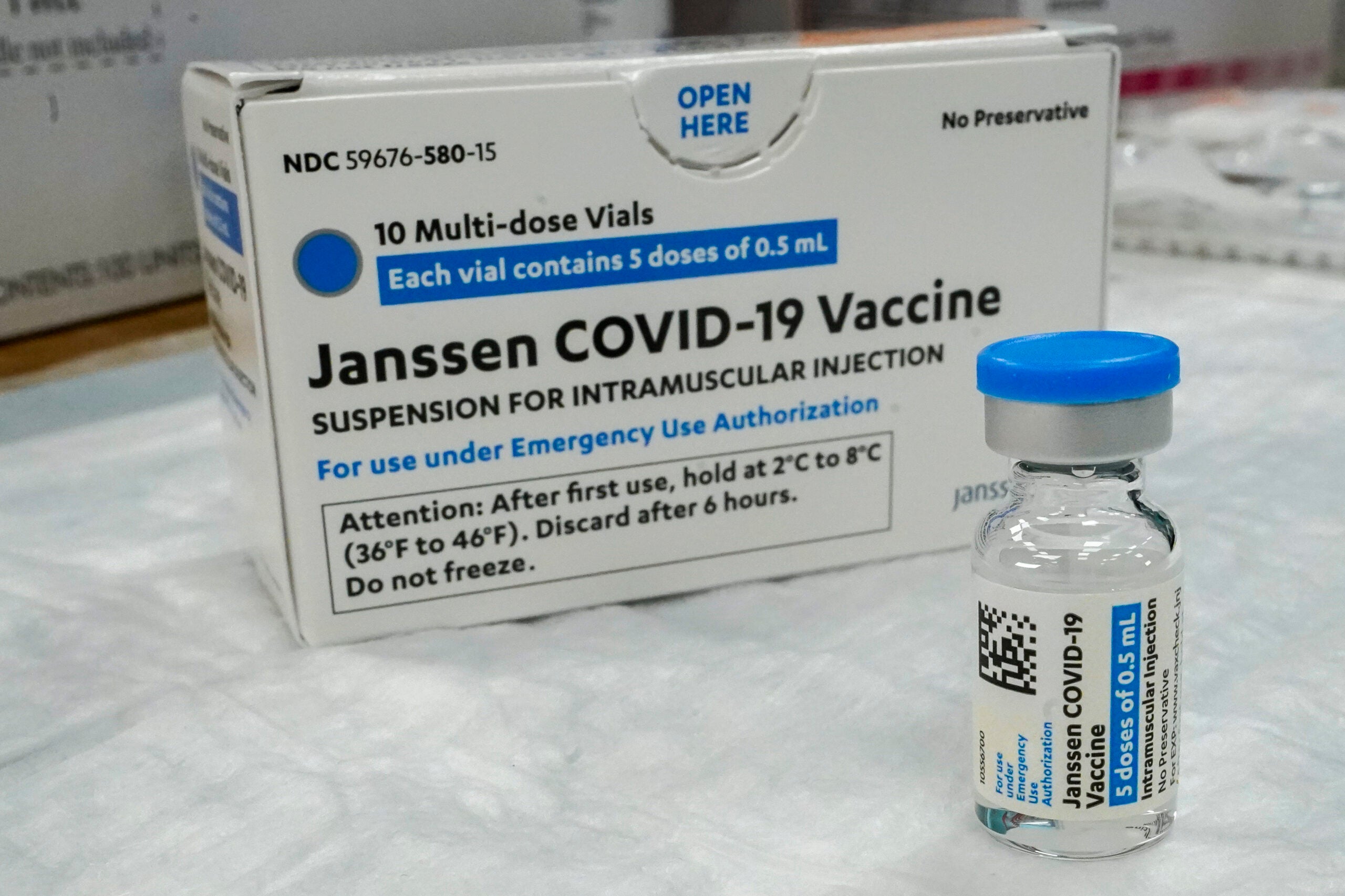
The Wisconsin Department of Health Services is instructing vaccine providers in the state to stop administering the Johnson & Johnson COVID-19 vaccine.
The Centers for Disease Control and Prevention and the Food and Drug Administration recommend a “pause” in the use of the vaccine, which is a single-dose, to investigate reports of potentially dangerous blood clots. The federal and state agencies said the request is out of an “abundance of caution.”
The FDA and CDC are reviewing six reports of a rare and severe blood clot found in women in the United States in the days after being vaccinated, in combination with reduced platelet counts. As of Monday, more than 6.8 million doses of the J&J vaccine have been administered in the U.S., according to the FDA, the vast majority with no or mild side effects. In Wisconsin, 160,080 doses of the Johnson & Johnson vaccine have been administered as of Monday.
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
During a media briefing Tuesday, Peter Marks, FDA director for the Center for Biologics, Evaluation and Research, said the blood clots appear to be “extremely rare,” but treatment is different from those used for other types of clots.
By not administering the vaccine, the CDC has time to determine if there are other cases of blood clots and has time to give health care providers information on how to treat it, according to DHS. A CDC committee will meet Wednesday to discuss the cases and the FDA has also launched an investigation, according to the Associated Press.
“Health care providers who see people presenting to them with either a low blood platelet count or blood clots should establish whether or not the individual has recently been vaccinated in order to inform the appropriate diagnostic evaluation and management,” said Marks.
“I know that the information we’re providing today is going to be very concerning to Americans who have already received that Johnson & Johnson or Janssen vaccine,” said CDC deputy director Anne Schuchat. “And I want to let you know what we’re doing to learn more and to protect people in the meantime. And what you can do to be on the alert.”
Health officials, including DHS Secretary Karen Timberlake, are stressing the reaction to the vaccine is extremely rare. Health officials are also pointing out that the Pfizer and Moderna COVID-19 vaccines remain safe. The Pfizer and Moderna vaccines are both mRNA vaccines and are different than the J&J vaccine.
“We are pausing administration of the Johnson & Johnson vaccine out of an abundance of caution,” Timberlake said in a press release announcing the state’s decision. “At this time, these adverse events appear to be extremely rare. Vaccine providers should not administer the Johnson & Johnson vaccine at this time, and should hold on to the vaccine until federal review has been completed.”
During a Tuesday press conference in Milwaukee, Gov. Tony Evers credited the FDA and CDC for being transparent about the adverse reactions related to the J&J vaccine and the quick pivot to give health systems more time to adjust treatment options. He said despite only six reported cases of blood clots, the news is likely to make some uneasy about getting vaccinated against the coronavirus.
“This is going to make it more difficult because people that are hesitant can use this as another way to make an excuse for not getting … that vaccine,” said Evers. “But I believe we can overcome that. It’s just going to take time and a lot of effort. We don’t change our hesitancy overnight.”
During a DHS media briefing Tuesday afternoon, Julie Willems Van Dijk, DHS deputy secretary, said around 156,000 doses of the J&J vaccine have been administered in Wisconsin and another 10,000 doses are on their way to the state.
“That is arriving this week as we speak,” said Willems Van Dijk. “We’ve notified our vaccine providers to accept that vaccine, to store it appropriately in refrigerators, to label it at this point in time: ‘do not use,’ to maintain appropriate temperatures and to hold it until a decision is made about this pause.”
Willems Van Dijk said the J&J vaccine has been used across the state with DHS focusing on getting it to local health departments. She said the agency isn’t aware of any severe clotting incidents happening to Wisconsinites and that people should keep adverse reactions in perspective.
“This risk of this rare event is about one in a million for this vaccine,” said Willems Van Dijk. “The risk of getting COVID in the United States is one in 10 … The risk of dying from COVID is one in 600 in the United States. And so as each of us thinks about this and weighs those risks, I think it’s really important to look at that whole picture.”
Vaccine providers should continue to properly store the vaccine and continue reporting “adverse events” to the Vaccine Adverse Events Reporting System (VAERS).
Dr. Ryan Westergaard, chief medical officer for DHS, said residents vaccinated with the J&J vaccine should contact their health care provider if they have a severe headache or new vision problems in the two weeks after receiving the vaccine. He also said people should monitor for three weeks after being vaccinated for a severe headache, abdominal pain, leg pain or shortness of breath.
Following federal guidance, some local health departments have announced they’ll temporarily stop administering J&J vaccines including departments in Bayfield, Dane, Douglas, Rock and Milwaukee counties. Essentia Health and Thedacare health systems also announced a pause.
A total of 3,572,358 doses of the coronavirus vaccine have been administered in Wisconsin as of Tuesday, with 70 percent of Wisconsinites age 65 and up fully vaccinated. As of Monday, 1,441,345 people in Wisconsin, or 24.8 percent of the population, have been fully vaccinated. Anyone in Wisconsin age 16 and up is eligible for a coronavirus vaccine.
According to the DHS, 922 new cases of COVID-19 and 10 new deaths were reported Tuesday in Wisconsin. The agency also reported 70 new hospitalizations.
The seven-day average of COVID-19 cases is 794, the highest it’s been since Feb. 13, when it was 806. The seven-day average has been on the rise since March 11, when it was at a low of 363 cases.
Wisconsin Public Radio, © Copyright 2025, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.