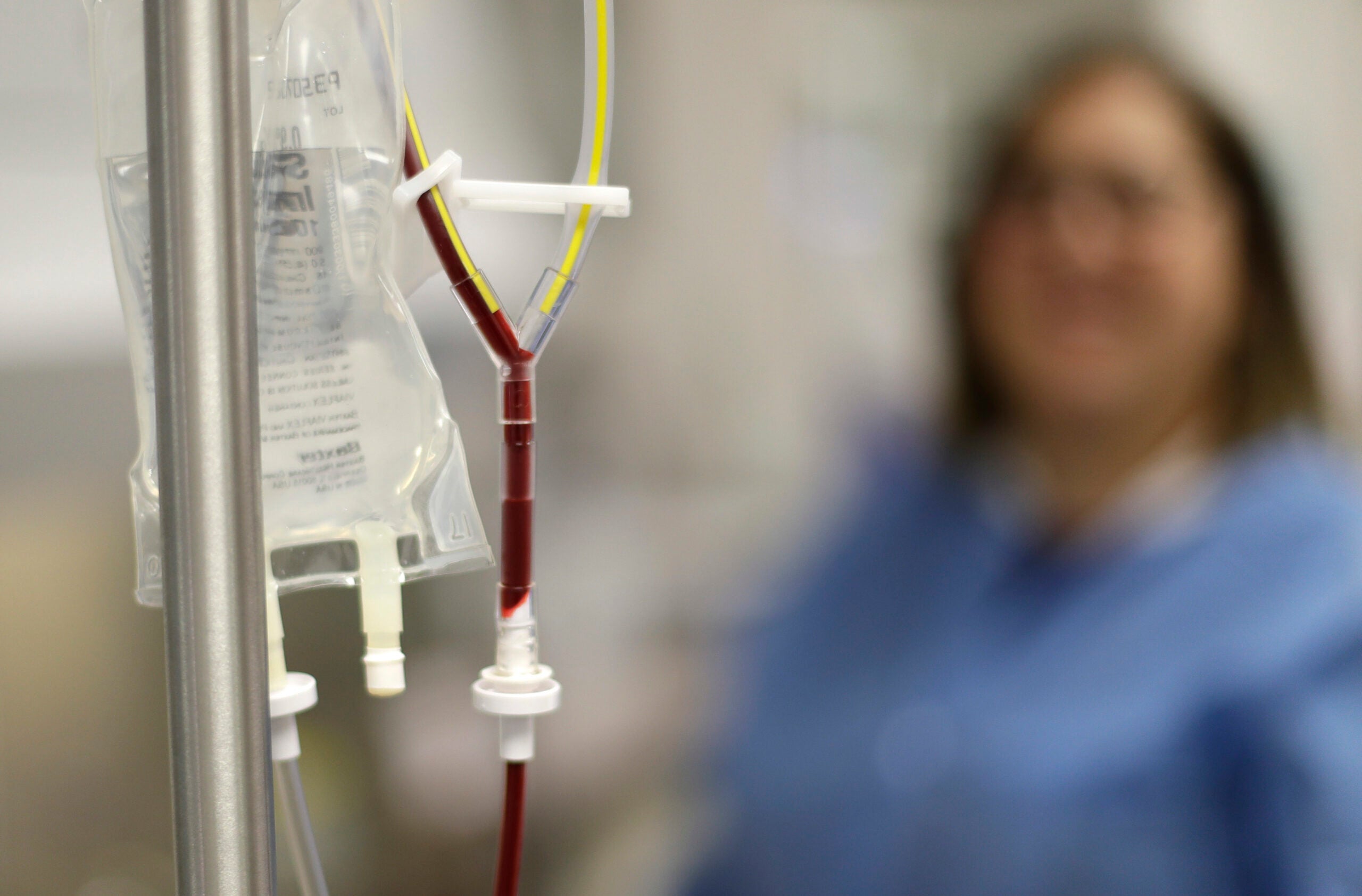
One way to fight cancer is by harnessing the patient’s own immune system to attack tumors. Medical researchers in Milwaukee are testing this emerging therapy, but with a twist.
In recent years, there’s been dramatic progress using a patients’ own immune cells to treat cancer. The U.S. Food and Drug Administration recently approved two therapies that use what are known as chimeric antigen receptors, or CAR-T cells. These are cells that are genetically modified to spot and attack tumors. Now the Medical College of Wisconsin has made its own version.
“We are hoping to advance on the science of CAR-T technology by targeting more than one cancer cell molecule. So the CAR-T clinical trial we have targets two molecules and we think this might be more effective for patients with certain types of blood cancers,” said Dr. Nirav Shah, leader the clinical trial.
Stay informed on the latest news
Sign up for WPR’s email newsletter.
One of the goals of the trail is to find out what the right dose of CAR-T cells is and whether some patients need to be re-treated.

Dr. Nirav Shah, left, leads a clinical cancer trial that helped Bret Carroll, right, who was diagnosed in 2011 with mantle cell lymphoma. Chuck Quirmbach/WPR
So far two people have participated in the clinical trial, and one is in remission. Bret Carroll, 52, entered the clinical trial in October after two unsuccessful bone marrow transplants for mantle cell lymphoma, which he was diagnosed with in 2011.
“So am I cured?” he said during a Thursday press conference at MCW. “I think that’s the answer that everybody wants. But only time will tell. I am cautiously optimistic on that.”
The MCW Clinical Cancer Center at Froedtert Hospital is offering the clinical trial for patients who have certain non-Hodgkin lymphomas. Doctors and researchers there are also hoping to get FDA approval for trials that will include children.
University of Wisconsin Hospital in Madison has treated two children with acute lymphoblastic leukemia using the recently approved therapy KYMRIAH. One child is very early in the process and another is in remission, said Dr. Mark Juckett, chief of bone marrow transplant at UW Carbone Cancer Center in Madison.
“What they’re doing at Froedtert Medical College of Wisconsin is actually pretty unique. Rather than giving the FDA approved products, they are creating their own product by using a novel virus that actually expresses proteins that allow the modified immune cell to see different targets on the cell.”
And he said there’s an economic advantage to MCW making the CAR-T cells on-site. The two FDA approved products cost around $400,000.
“That’s obviously not the driver here (for MCW doing this) but we all live in a world where costs are meaningful and to make cells on site is unique and interesting. So the approach they’re taking is valuable from a couple of different perspectives,” Juckett said.
CAR-T cells been developed and used at a number of centers including the University of Pennsylvania, Memorial Sloan Kettering Cancer Center, and the National Institutes of Health.
While Steven Rosenberg, chief of surgery at the National Cancer Institute’s Center for Cancer Research, says the different forms of adoptive cell transfer “are still being developed,” after several decades of painstaking research, the field has reached a tipping point.
“In the next few years,” Rosenberg said, “I think we’re going to see dramatic progress and push the boundaries of what many people thought was possible with these adoptive cell transfer–based treatments.”
Wisconsin Public Radio, © Copyright 2024, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.