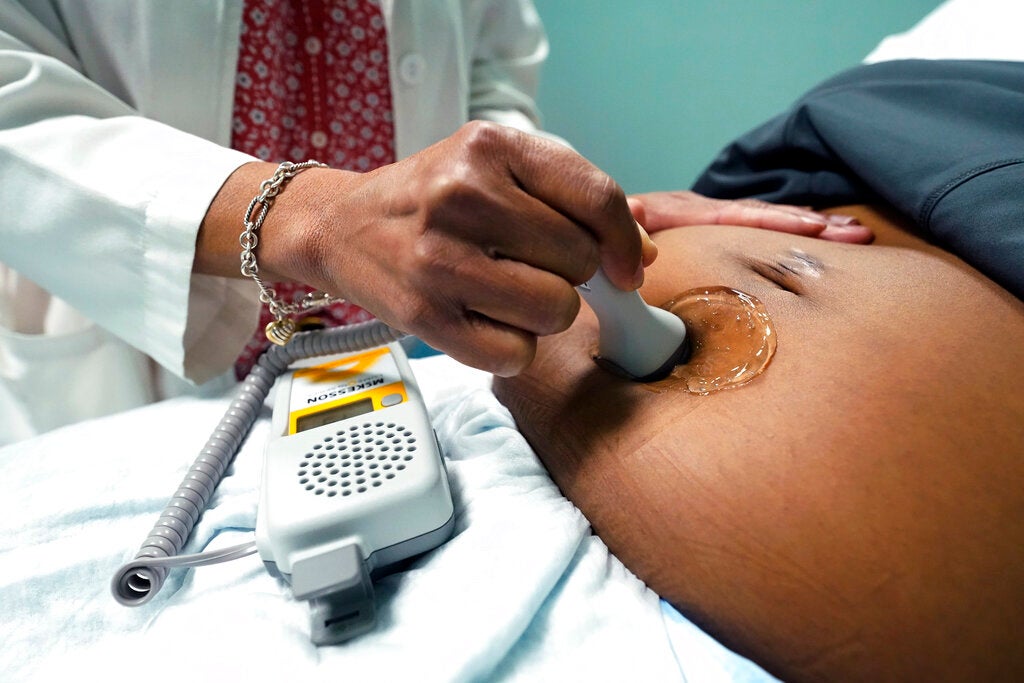
The placenta is key to a healthy pregnancy — providing all the nutrients and oxygen a growing fetus needs. But when the placenta doesn’t function the way it should, a pregnancy can end in miscarriage or premature birth. And there isn’t much doctors can do.
Placental research is still relatively new, and it’s unclear what causes an unhealthy placenta to form. But research out of the University of Florida has narrowed in on a protein key to a functioning placenta — and researchers there are collaborating with the University of Wisconsin-Madison to bring potential therapies closer to human clinical trials.
Helen Jones, an associate professor at the University of Florida, said boosting that protein via gene therapy can make a placenta function properly.
News with a little more humanity
WPR’s “Wisconsin Today” newsletter keeps you connected to the state you love without feeling overwhelmed. No paywall. No agenda. No corporate filter.
“A lot of these complicated pregnancies have lower amounts of the protein that’s made, so the models we use also have lower amounts of these factors,” Jones said. “We’re basically giving the placenta the ability to make more and make the right amount of these (proteins).”
Jones led studies involving both mice and guinea pigs that were successful in replacing the missing protein and restoring function to the placenta.
Now a study from the Wisconsin National Primate Research Center at UW-Madison has shown the therapy is safe in rhesus macaques.
Jones and Jenna Schmidt, who led the Wisconsin study, recently spoke to WPR’s “Wisconsin Today” about what they’ve found and what they hope to see in future studies.
The following was edited for clarity and brevity.
An ‘overlooked’ organ
Helen Jones: None of us would be here without the placenta that we had when we were fetuses. It maintains the environment of the uterus during pregnancy. It acts as the main communication hub between the pregnant individual and the fetus, and it supplies everything that’s needed for growing that fetus.
Jenna Schmidt: The placenta is all fetal tissue. It serves to exchange oxygen, give nutrients to the (fetus) and remove waste. It’s so important to early pregnancy, but it also can program the child’s health throughout their lifespan. I think it’s one of the most overlooked organs, and it’s so vital to each one of us and how we are here today.
Findings so far
HJ: We’re replacing a protein that we know is missing in a lot of complicated pregnancies. It is a protein named “insulin-like growth factor 1,” or IGF-1. Placentas that aren’t working very well don’t make as much of this protein as they should, and so we’re replacing it and helping the placentas make the right amount.
To do that, we deliver a nanoparticle containing the gene for this protein to the placenta. The cells of the placenta are the only ones that can actually read the instructions that we put inside this nanoparticle, so they then learn to make the protein. Studies so far have demonstrated that we’re restoring the function of the placenta, and in that we’re able to restore the normal growth of the fetus itself.
JS: We saw that the delivery mechanism was working safely. What we’re currently trying to do is deliver this at a time point that would be similar to about the third trimester in human pregnancy and then extend treatment all the way to term and see what the safety and efficacy of the treatment is.
When a placenta is unhealthy
HJ: If a placenta is not formed properly or not working properly, it can impact the birth weight at delivery and can also go on to impact lifelong health, as well. With placenta issues later in pregnancy, you’re more likely to get a smaller baby, who would then need to be delivered early so that they can go to the NICU (neonatal intensive care unit) to continue growing. There’s lots of associations between being born too small and developing cardiovascular or metabolic complications in later life. If issues arise very early in pregnancy, you end up potentially losing the pregnancy. Other problems such as preeclampsia can significantly impact pregnant individuals.

Working up the ‘species ladder’
HJ: We’ve been working on this project for about 14 years now. It does sound like a long time. But realistically, getting something all the way to the bedside is more like a 25-year process.
We’ve been working our way through our animal models and our models of (donated) human placenta that we use in the lab in pots. Our animal models started with the mouse and moved to the guinea pig. A guinea pig placenta is much more reflective of the human’s than the mouse is. Also, guinea pigs have much longer pregnancies, so we’re able to have a treatment where we go in and then improve that placental function. This treatment is given across the second half of pregnancy, similar to the time frame we would aim for in humans. Then, we were able to restore normal growth by birth. We know now that we have to give multiple treatments to have that impact.
We’ve sort of worked our way up the species ladder, so we’re now able to be testing this in the primates. We need to find out if giving multiple treatments would be safe. It is safe in the guinea pig, but now working with the non-human primate will really tell us if that safety holds up.
JS: (Rhesus macaques) are a great animal model for human pregnancy because they have so many similar features in terms of their placentas and their developmental stages in pregnancy. We’ll be looking to see if the treatment can be developed into a safe delivery strategy for human clinical trials.
The optimal goal would be to deliver it into mom’s blood through an IV in her arm, and the particle would specifically target the placenta. There’s a couple additional developments that we need to do to get there, but it’s looking pretty promising when we deliver it right to the placenta.
‘Underfunded’ research leads to slow progress
HJ: Historically, women’s health has been extremely underfunded and under-investigated. Along the way, we’ve had to develop the mechanisms that we use to actually do our research, to deliver the treatment to the placenta (because) that’s not a normal target for drugs.
While the placenta is indeed inside that pregnant woman, it actually belongs to the fetus. Therefore, this is impacting all of humankind, not just women. There’s a lack of funding, lack of understanding and a little bit of a lack of bravery.
Wisconsin Public Radio, © Copyright 2025, Board of Regents of the University of Wisconsin System and Wisconsin Educational Communications Board.